A major challenge in chemistry and biology is the ability to identify biomolecules, such as enzymes, in their functionally active state and to deliberately control their behavior, particularly in complex environments.
Sensing biomolecular activity, rather than simply measuring concentration, is essential for understanding biological processes and for early disease diagnosis. Our work focuses on developing nanoscale technologies that can enable activity-based sensing. These approaches can directly report on enzymatic function, enabling quantitative measurements for the early detection of diseases such as cancer.
Programming biomolecular function requires tools that can actively modulate activity with precision. Small-molecule drugs and protein regulators typically act through single binding sites or fixed interfaces, which limits control over spatial organization and local chemical environment. In contrast, nanoscale systems are often comparable in size to biomolecules and can be engineered to engage enzymes and proteins through multiple, spatially defined interactions simultaneously. This enables control over enzyme orientation, local concentration, and microenvironment in ways that are difficult to achieve with small molecules or proteins alone.
By integrating biological building blocks – DNA, peptides, and proteins – with synthetic nanomaterials, our lab develops hybrid systems that both sense biomolecular activity and program biomolecular function. Our work aims to establish design principles at the nano–bio interface to enable applications in biosensing, biocatalytic synthesis, synthetic biology, and therapeutic biomolecular systems.
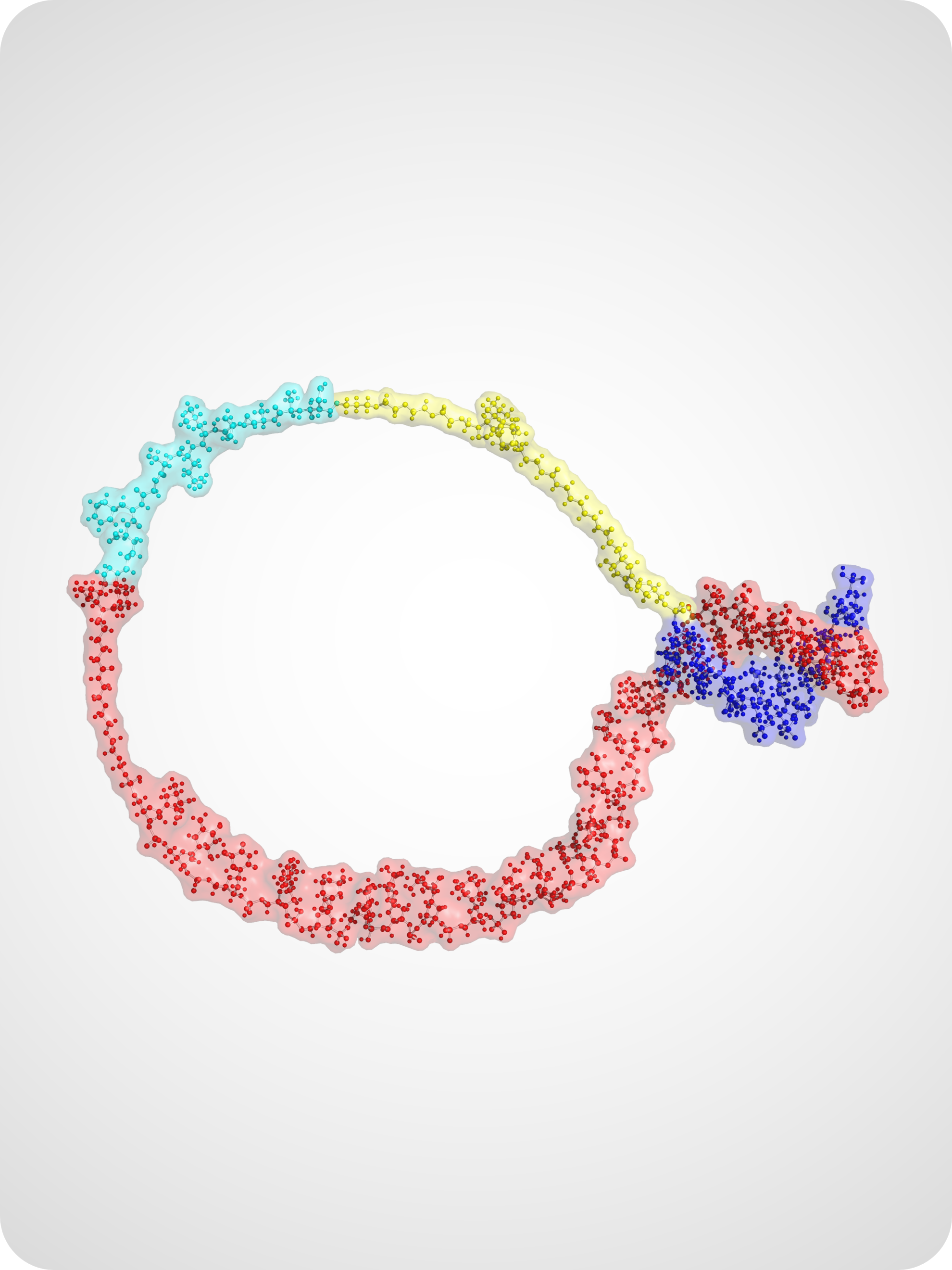
Activity-Based Sensing of Biomolecules
Our lab designs sensing technologies that directly report on biomolecular activity rather than abundance. By integrating DNA nanotechnology with enzymatic reporters, plasmonic nanostructures, and CRISPR-based amplification, we create sensitive and quantitative platforms for detecting nucleic acids, proteases, and other biologically relevant targets.
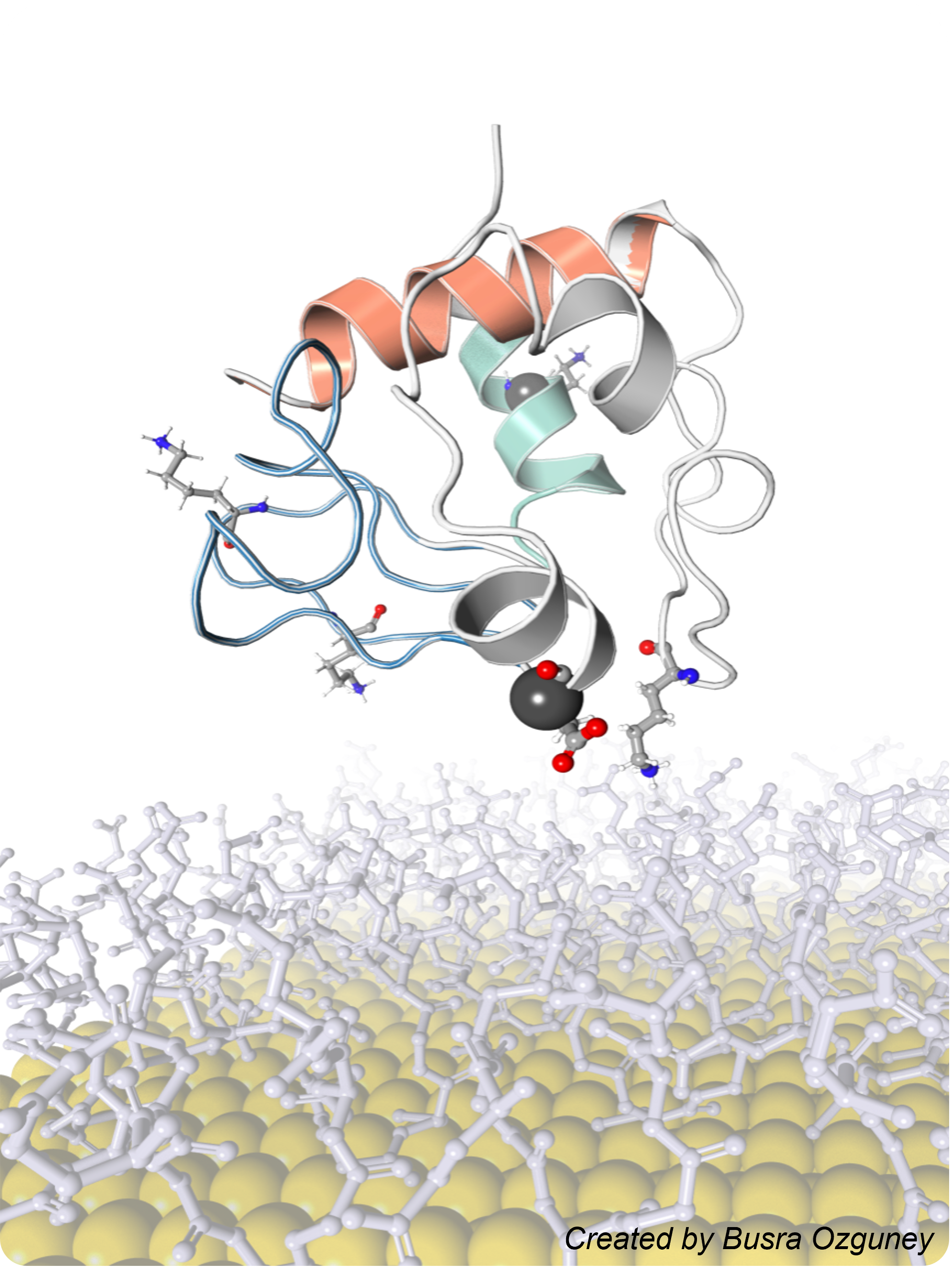
Sequence-Encoded Nanomaterial Interfaces for Enhancing Enzyme Biocatalysis
We engineer nanomaterials with sequence-defined surface ligands that can bind enzymes with high affinity, enhance their catalytic activity by more than 20-fold, and even improve product selectivity – all without requiring any covalent or genetic modification of the enzyme. This work can open new opportunities in biosensing, biocatalytic synthesis, and therapeutic enzyme applications.
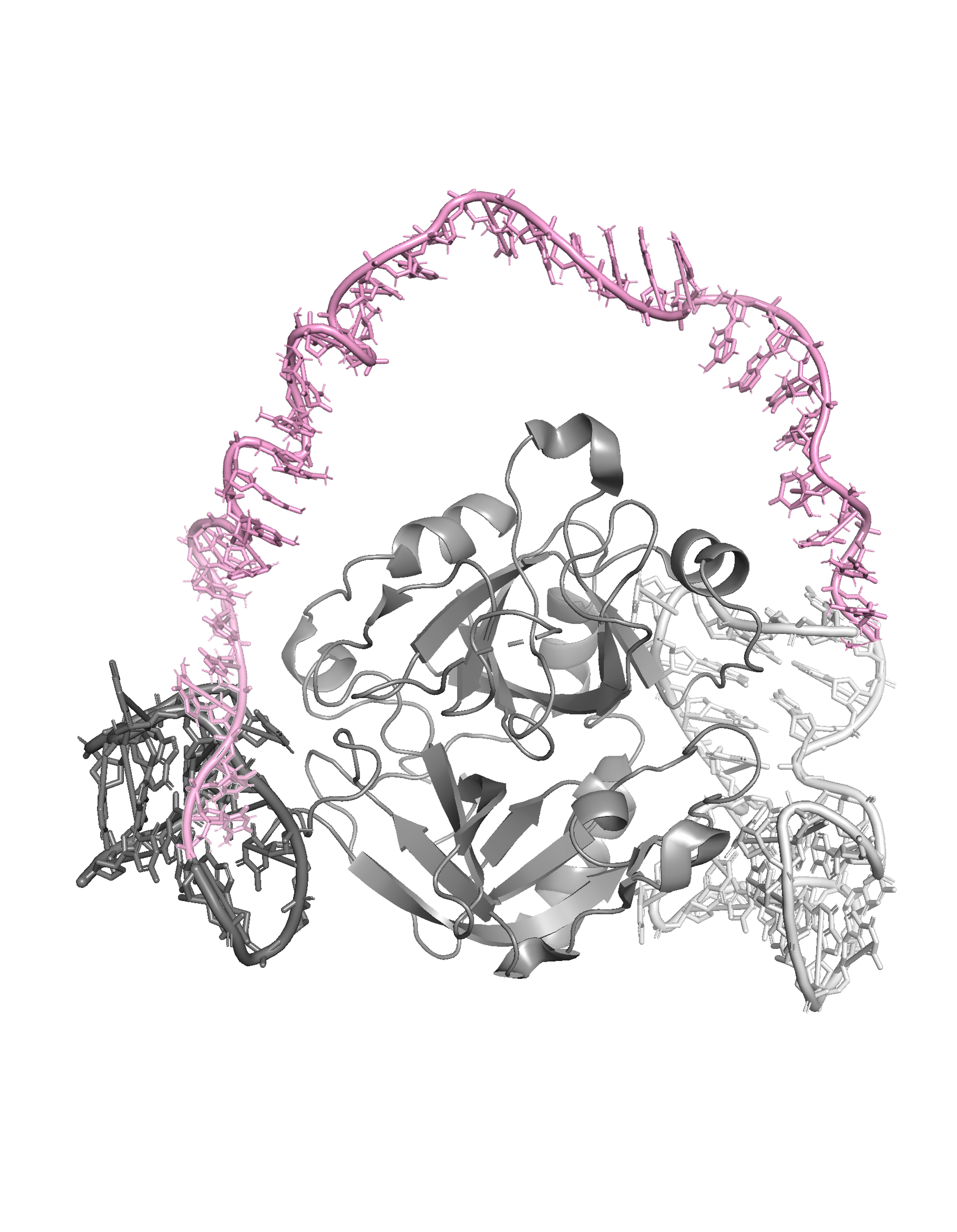
Designer DNA Switches for Allosteric Control of Enzymes
We develop single-molecule DNA-based switches that enable programmable, allosteric control ON/OFF control of enzyme activity in response to user-defined molecular cues. This work provides a new framework for dynamic regulation of enzymes, with applications in biosensing, synthetic biology, and controllable biocatalytic systems.
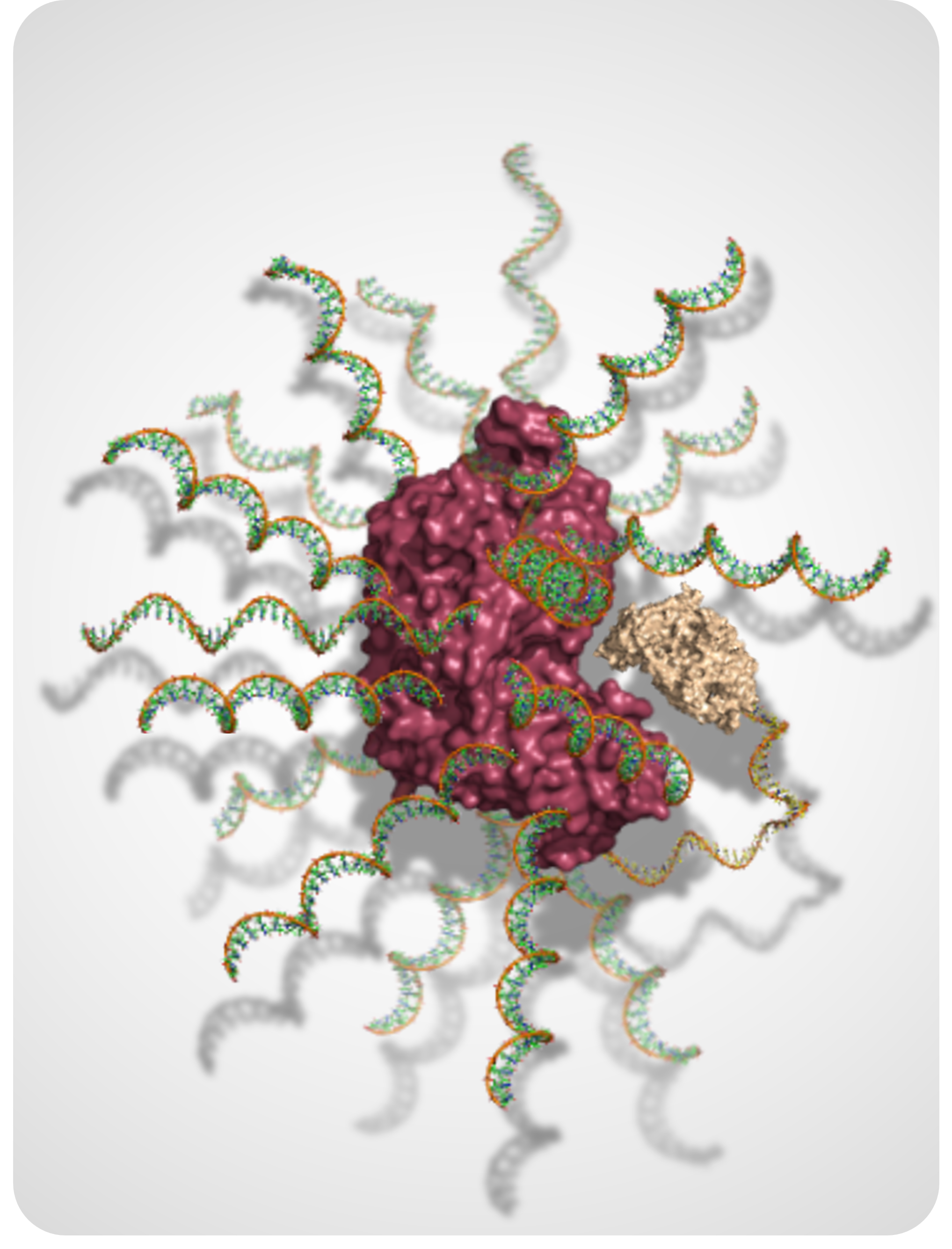
DNA-Based Nanostructures for Biomolecular Stability, Delivery, and Therapeutic Design
We use programmable DNA architectures to protect, organize, and deliver functional biomolecules. This research explores how nucleic acid scaffolds and oligonucleotide design principles can be leveraged to improve stability, control molecular behavior in complex environments, and enable next-generation oligonucleotide and biomolecular therapeutics.

